Tennessine: A Journey to the Limits of the Periodic Table
Related Articles: Tennessine: A Journey to the Limits of the Periodic Table
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Tennessine: A Journey to the Limits of the Periodic Table. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Tennessine: A Journey to the Limits of the Periodic Table
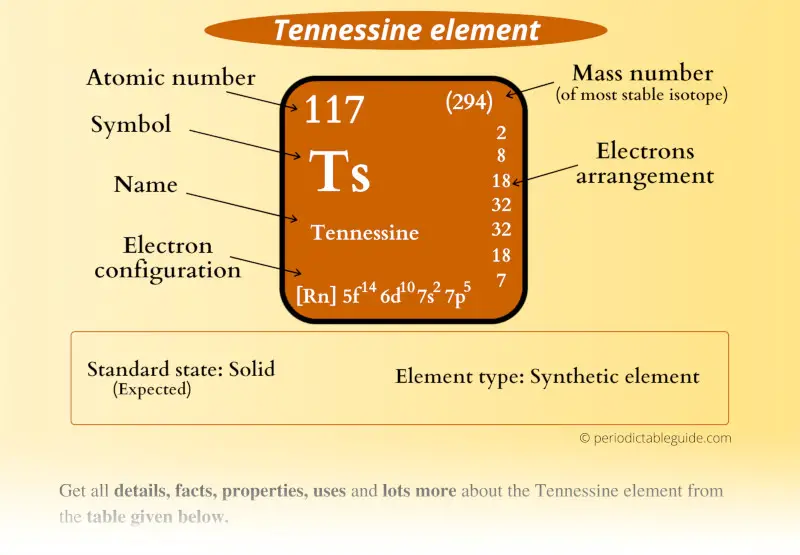
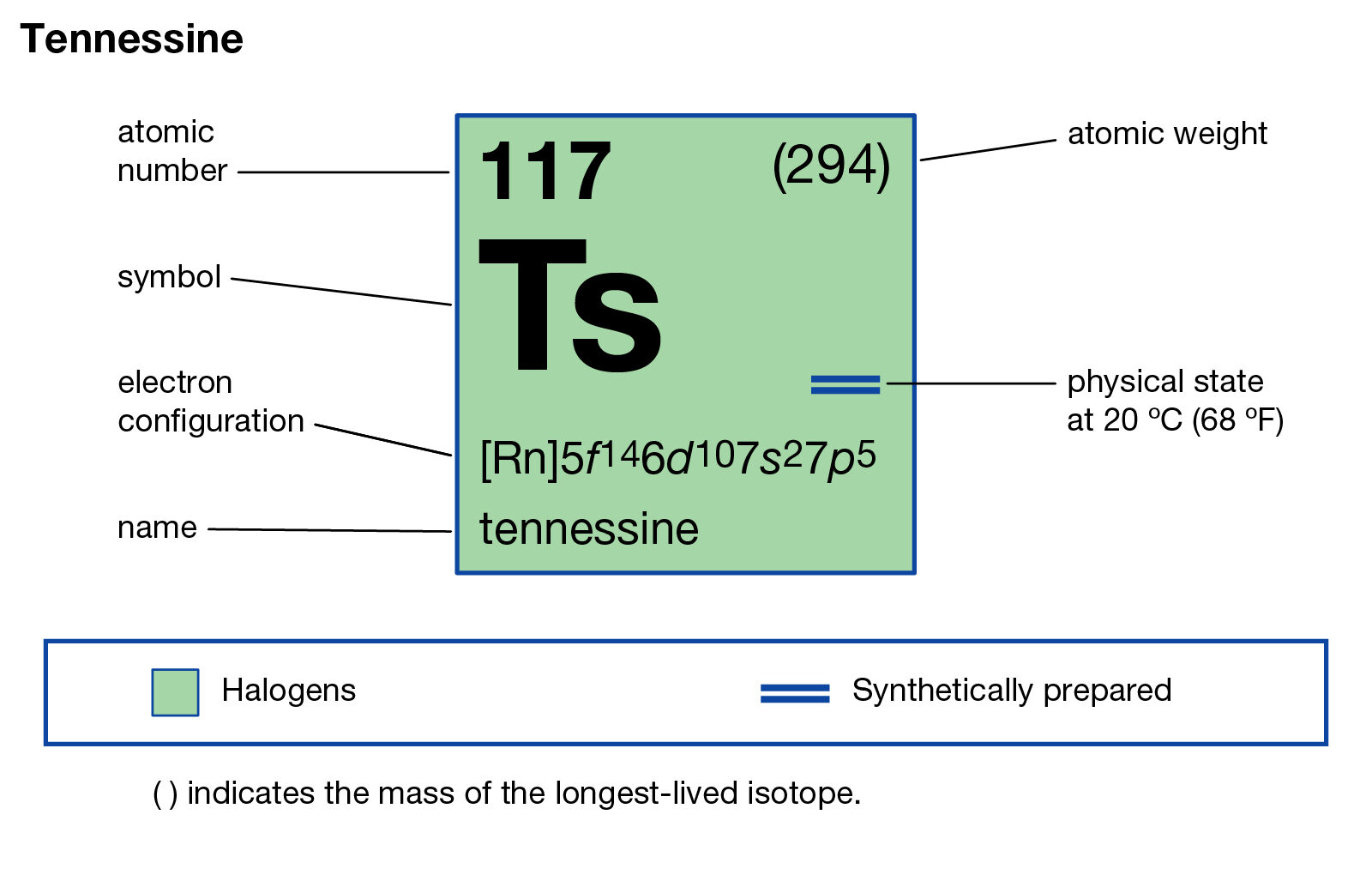
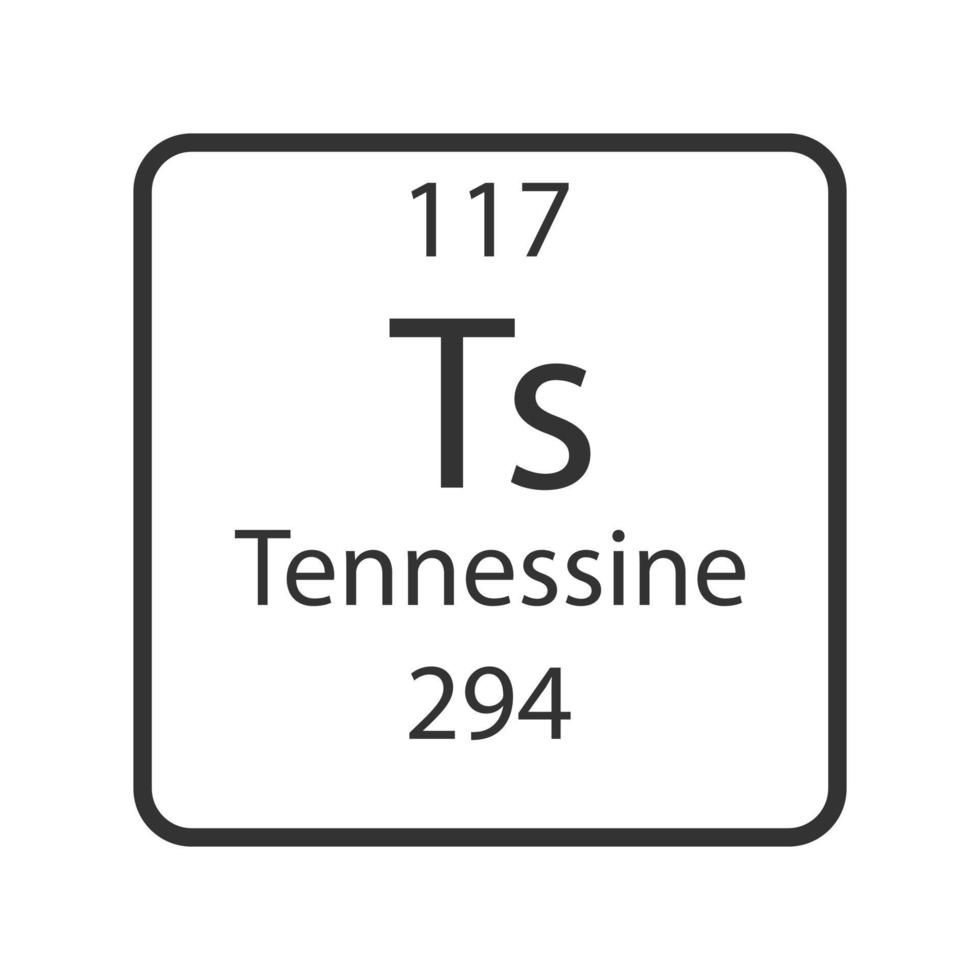
Tennessine (Ts), a synthetically produced element with atomic number 117, stands as a testament to the relentless pursuit of scientific discovery. Its existence, though fleeting and challenging to study, offers a unique window into the fundamental nature of matter and the intricate workings of the atomic nucleus. This article delves into the history of Tennessine’s discovery, its intriguing properties, and the challenges and opportunities presented by its study.
The Quest for New Elements:
The periodic table, a cornerstone of chemistry, has been a source of both fascination and inspiration for centuries. Its structure, with its predictable trends and patterns, has guided countless scientific endeavors. However, the quest for new elements, especially those residing at the fringes of the table, has always been a formidable challenge.
Tennessine, a superheavy element belonging to the halogen group, was first synthesized in 2010 at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, in collaboration with scientists from the Lawrence Livermore National Laboratory (LLNL) in the United States. The discovery was a culmination of years of dedicated research and sophisticated experimental techniques.
A Tale of Fusion and Decay:
The creation of Tennessine involved a process known as nuclear fusion, where two atomic nuclei are forced to collide and fuse together, forming a heavier nucleus. In this case, the researchers bombarded a berkelium-249 target with calcium-48 ions. The resulting fusion reaction produced a few atoms of Tennessine-294, which subsequently decayed into lighter elements through a series of alpha-particle emissions.
The short-lived nature of Tennessine, with a half-life of just 78 milliseconds, posed significant challenges for its study. The researchers had to rely on sophisticated detectors and advanced data analysis techniques to confirm its existence and characterize its properties.
Unveiling the Properties of Tennessine:
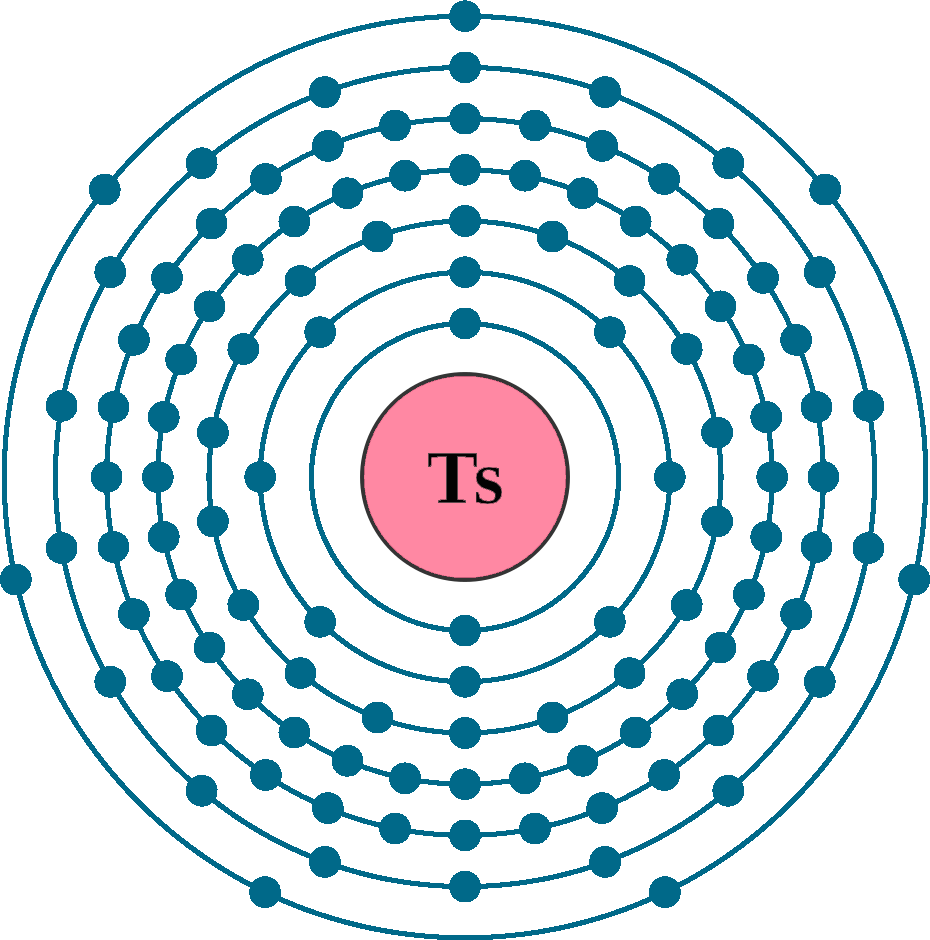
Despite its fleeting existence, scientists have been able to glean valuable insights into the behavior of Tennessine. Its position in the periodic table, as a halogen, suggests that it should exhibit similar chemical properties to its lighter counterparts, such as iodine and astatine. However, the extreme size of its atom and the strong influence of relativistic effects (which arise from the high speeds of electrons in heavy atoms) are expected to significantly alter its chemical reactivity.
Theoretical calculations predict that Tennessine should be a solid metal at room temperature, though its exact properties are yet to be experimentally confirmed. Its chemical behavior is likely to be influenced by its electronic structure, which is still being explored.
The Significance of Tennessine:
The discovery and study of Tennessine hold profound implications for our understanding of the universe. It pushes the boundaries of our knowledge about the limits of the periodic table and provides valuable insights into the stability and behavior of superheavy elements.
Moreover, the techniques developed for synthesizing and studying Tennessine have opened new avenues for research in nuclear physics and chemistry. The insights gained from these studies can contribute to the development of new materials and technologies, potentially leading to advancements in various fields, including medicine, energy, and materials science.
FAQs about Tennessine:
Q: Is Tennessine a naturally occurring element?
A: No, Tennessine is a synthetic element, meaning it is not found naturally on Earth. It is produced in laboratories through nuclear fusion reactions.
Q: What are the potential applications of Tennessine?
A: Due to its short half-life and limited availability, Tennessine currently has no practical applications. However, the research on its properties could contribute to the development of new materials and technologies in the future.
Q: How is Tennessine named?
A: Tennessine is named after the state of Tennessee, which is home to the Oak Ridge National Laboratory, where researchers played a significant role in its discovery.
Q: What are the challenges of studying Tennessine?
A: The primary challenge in studying Tennessine is its short half-life, which makes it difficult to produce and observe. Additionally, the extreme conditions required for its synthesis and the limited amount of available material pose significant experimental challenges.
Tips for Understanding Tennessine:
- Visualize the periodic table: Familiarize yourself with the position of Tennessine within the table and its relationship to other elements.
- Explore the concept of nuclear fusion: Understand how fusion reactions are used to create new elements, like Tennessine.
- Learn about the challenges of studying superheavy elements: Appreciate the unique difficulties associated with researching elements with short half-lives.
- Consider the potential applications of Tennessine: Think about how the knowledge gained from studying this element could contribute to future advancements.
Conclusion:
Tennessine, a synthetically produced element residing at the edge of the periodic table, represents a remarkable achievement in scientific exploration. Its discovery has broadened our understanding of the nature of matter and the intricacies of the atomic nucleus. While its practical applications remain limited, the insights gained from its study hold immense potential for future advancements in various fields. Tennessine serves as a reminder of the boundless possibilities of scientific inquiry and the enduring human desire to push the boundaries of knowledge.



![]()
Closure
Thus, we hope this article has provided valuable insights into Tennessine: A Journey to the Limits of the Periodic Table. We hope you find this article informative and beneficial. See you in our next article!